The ongoing Inspire-T human cohort is designed to specifically study this new concept of healthy aging trajectories focused on integrated clinical and biological markers, to understand the underlying biological mechanisms of maintenance of key functions, and to contribute to the validation of biological age measures.
The Inspire-T cohort consists of 1,086 women and men aged from 20 to 102 years at the time of recruitment. Participants were recruited in the Toulouse region of France, and had varying levels of functional status at baseline. We opted to not use too stringent eligibility criteria; only persons with severe diseases compromising life expectancy at 5 years (or at 2 years for frail older subjects and those aged 80 years or older) and persons who were legally incapable were excluded. Participants were stratified into ten-year age groups, with oversampling of people older than 70 years.
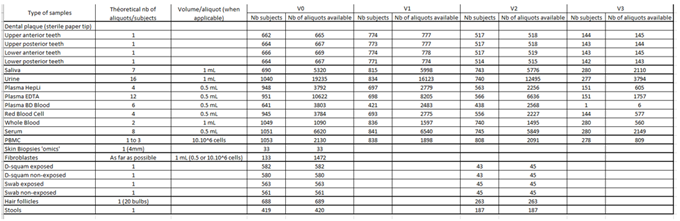
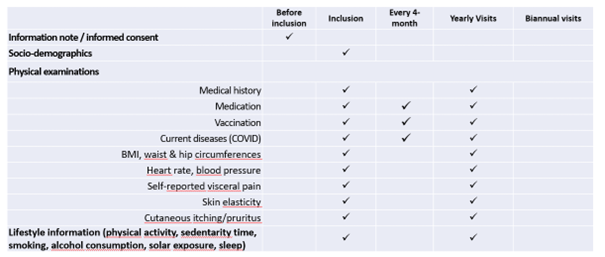
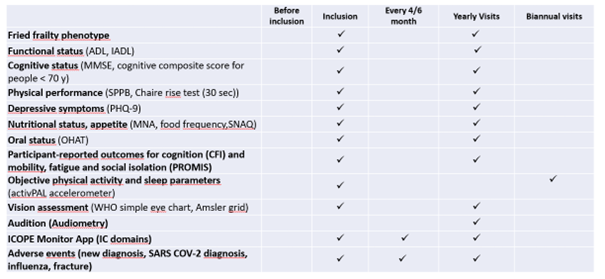
Clinical data and biospecimens were collected at baseline and then annually at the research facilities or the participant’s at home. A core set of questionnaires covering an extensive spectrum of domains including social and demographic measures, health status (including medication, chronic conditions, autonomy, Fried frailty criteria, falls, nutritional status) and functioning, cognitive, psychological measures, use of the health care system, and several lifestyle factors (including smoking, alcohol consumption, physical activity, sedentarism, sleep, and dietary intake) was adopted. Intrinsic capacity and its domains were assessed according to the recommendations included in the WHO ICOPE program. A cognitive composite score combining four cognitive tests (free and total recall of the Free and Cued Selective Reminding test, ten Mini-Mental State Examination orientation items, Digit Symbol Substitution Test, and Category Naming Test) was computed in subjects less than 70 years old. Additional physiological parameters including whole body/brain MRI, DEXA, oxygen consumption test (VO2 max) and muscle strength test (Cybex) were offered to a sub-sample of the subjects at baseline (MRI, VO2 max, strength test) and every two years (DEXA). Every four months for the first year, then every six months from the second year of monitoring, participants were asked to assess their functions at home using the ICOPE MONITOR application. Depending on the results observed, a more in-depth evaluation could be proposed in order to seek the potential causes of decline (including biological, social, environmental causes, intercurrent diseases) and propose targeted preventive strategies and personalized interventions. Biospecimens including approximately 60 ml of fasting blood (including PBMC, serum, lithium heparinized plasma, EDTA plasma, whole blood), urine, saliva, dental biofilm, are collected from all subjects at baseline and annually thereafter. Nasopharyngeal swabs, cutaneous surface samples, stools, hair follicles, and skin biopsy, are collected optionally at the baseline visit. Aliquots of biological material are stored at -80°C (whole blood, serum, plasma, urine, dental biofilm, saliva, skin biopsy, fibroblasts, stools and hair follicles) or at -196° C liquid nitrogen (PBMC) at the central lab (CRB TBR, CHU Toulouse/ IFB PURPAN, Toulouse, France).
Trial plan
BIiobank (update on January 2024)